
ELISA Kits
- • Anemia ELISA kits
- • Allergy ELISA kits
- • Autoimmune Disease kits
- • Bone Metabolism ELISA kits
- • Blood bank ELISA kits
- • Cancer ELISA kits
- • Cardiac Markers ELISA kits
- • Diabetes Assays ELISA kits
- • Drug test ELISA kits
- • Fertility ELISA kits
- • Food ELISA kits
- • Infectious Disease ELISA kits
- • Other ELISA Kits
- • Parasitology ELISA kits
- • Steroid ELISA kits
- • Thyroid ELISA kits
Rapid Tests
- • Allergy Rapid tests
- • Bone Metabolism
- • Cancer Rapid tests
- • Cardiac markers Rapid tests
- • Drug Tests
- • Fertility Rapid tests
- • Hepatitis Panel
- • Infectious Disease & other tests
- • Other
- • Ovulation Rapid tests
- • Pregnancy tests
- • Urine Reagent Strips tests
IFA Kits
Chemiluminescence Immuno Assays
- • Allergy Assays
- • Autoimmune Thyroid Assays
- • Cardio-Vascular Monitoring
- • Diabetes Assays
- • Fertility Assays
- • Growth Deficiency
- • Infectious Disease Assays
- • Others
- • Steroid Assays
- • Thyroid Assays
- • Tumor Marker Assays
Serology Tests
- • ASO (Anti-Streptolysin-O)
- • CRP (C-Reactive Protein)
- • Mono (Infectious Mononucleosis)
- • RF (Rheumatoid Factor)
- • RPR (Rapid Plasma Reagin)
- • SLE (Systemic Lupus Erythematosus)
Instrumentation



HIV 1/2 Rapid Test (Serum,WB,Plasma)
| Name |
HIV 1/2 Serum,WB, Plasma (Cassette) RapiCard InstaTest |
|---|---|
| Full name |
Human HIV 1/2 Serum,WB, Plasma (Cassette) RapiCard InstaTest, Export Use Only |
| Category Name | Pregnancy tests |
| Test | Bulk Or Boxed in 40 Tests |
| Specificity | 100 % |
| Sensitivity | 99 % |
 |
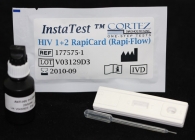 |
 |
HIV 1/2 Rapid Test (Serum,WB,Plasma) description:
"EXPORT USE ONLY"
HIV 1/2 Rapid Test (Cassette) is a single use rapid test device for qualitative detection of antibodies to Human Immunodeficiency Viruses (HIV) in whole blood, serum, and plasma samples.
Materials provided with the test kits
Test Cassettes , Droppers , Buffer Package insert
Materials required but not provided
Specimen collection containers,Timer,Centrifuge
HIV 1/2 Rapid Test Kit Background Information
The Human Immunodeficiency Viruses type 1 and type 2 are etiological agents of the acquired immunodeficiency syndrome (AIDS). HIV has been isolated from patients with AIDS, AIDS related complex (ARC) and from healthy individuals at high risk for (AIDS). Infection with HIV is followed by an acute flu-like illness. This phase may remain unnoticed and the relationship to HIV infection may not be clear in many cases. The acute phase is typically followed by an asymptomatic carrier, which progresses to clinical AIDS in about 50% of infected individuals within 10 years after seroconversion. Serological evidence of HIV infection may be obtained by testing for HIV antigens or antibodies. Antigen to HIV can be detected throughout virtually the total infection period, starting at or shortly after the acute phase and lasting until the end stage of AIDS. Therefore, the use of highly sensitive antibody assays is the primary approach in serodiagnosis of HIV infection.
HIV 1/2 Rapid Test Principle
This HIV (1+2) rapid test device employs chromatographic lateral flow device in a cassette format. Colloidal gold conjugated recombinant antigens (Au-Ag) corresponding to HIV-1 gp120, gp41 and HIV-2 gp-36 are dry-immobilized at the end of nitrocellulose membrane strip. HIV 1+2 antigens are bond at the Test Zone (T) and rabbit anti-HIV 1+2 monoclonal antibodies are bond at the Control Zone (C). When the sample is added, it migrates by capillary diffusion rehydrating the gold conjugate. If present in sample, HIV antibodies will bind with the gold conjugated antigens forming particles. These particles will continue to migrate along the strip until the Test Zone (T) zone where they are captured by the HIV 1+2 antigens generating a visible red line. If there are no HIV 1 or 2 antibodies in sample, no red line is formed in the Test Zone (T).The gold conjugate will continue to migrate alone until it is captured in the Control Zone(C) by the rabbit anti-HIV 1+2 antibodies aggregating in a red line, which indicates the validity of the test.
HIV 1/2 Rapid Test Kit Performance and Characteristics
In a clinical evaluation of the performance of Diagnostic Automation HIV 1/2 Rapid Test using 2657 confirmed negative and 667 positive samples, the resuls are as follow:
Sensitivity 99.4% (666/667)
Specificity 100% (2657/2657)
Accuracy 99%
Overall agreement 100% with reference ELISA tests
Limitations of HIV 1/2 Rapid Test Kit
1. Negative results do not exclude the possibility of HIV exposure or infection. Infection through recent exposure (seroconversion) to HIV may not be detectable. A test giving an invalid result should be repeated. This HIV (1+2) Rapid Test does not differentiate between recognition of HIV-1 and HIV-2 antibodies.
2. If after retesting of the initially reactive samples, the test results are negative, these samples should be considered as non-repeatable (false positive) and interpreted as negative. As with many very sensitive rapid diagnostic tests, false positive results can occur due to the several reasons, most of which are related but not limited to the quality of the sample and exposure of the test to humidity. Please contact Diagnostic Automation technical support for further assistance 818-591-3030.
For more information about Rapid Tests, ELISA Kits, IFA Kits, CLIA Test Kits, or Serology tests, please see our website home page, or contact our Customer Service Representative at 818-591-3030.
*Not to be sold, shipped, or distributed in the United States.
ELISA kits - Rapid tests- Drug tests- Pregnancy test - IFA kits - CLIA assays - Serology tests - Instrumentation
©1992 Diagnostic Automation/Cortez Diagnostics Inc. All rights reserved.


 HCG Combo Rapid Test (Urine/Serum) (Strip) (5mm)
HCG Combo Rapid Test (Urine/Serum) (Strip) (5mm)
 HCG Combo Urine/Serum Rapid Test (Strip) (3.5mm)
HCG Combo Urine/Serum Rapid Test (Strip) (3.5mm)
 HCG Urine Rapid Test (Cassette) Self Testing
HCG Urine Rapid Test (Cassette) Self Testing
 HCG U/S Rapid Test (Cassette)
HCG U/S Rapid Test (Cassette)
 HCG Midstream Urine Rapid Test (Cassette) Self Testing
HCG Midstream Urine Rapid Test (Cassette) Self Testing








